

|
|||
|
|||
Molecular Function of NCAPP1
|
|||
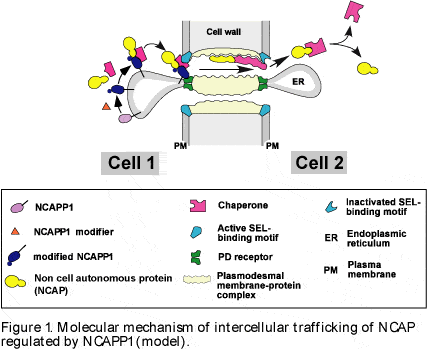
| Plasmodesmata play an important role in regulating intercellular trafficking of non-cell-autonomous proteins (NCAPs). We isolated a putative component of this NCAP pathway, NtNCAPP1, from tobacco tissues using affinity chromatography methods based on interaction with CmPP16, a demonstrated NCAP involved in the intercellular trafficking of RNA (Lee et al., 2003, Figure 1). | |||
 |
|||
| Deletion of the ER-targeting trans-membrane domain from NtNCAPP1 caused it to function as a gain-of-function dominant negative mutant when expressed in tobacco. This mutant form could block the trafficking of some, but not all, NCAPs, suggesting selectivity of NCAPP1 function. NtNCAPP1 encodes a putative ER-targeted trans-membrane protein with homology to aldose-1-epimerase (mutarotase) that is involved in galactose metabolism in E.coli . However, whether this enzymatic activity is required for NtNCAPP1 function remains to be shown. | |||
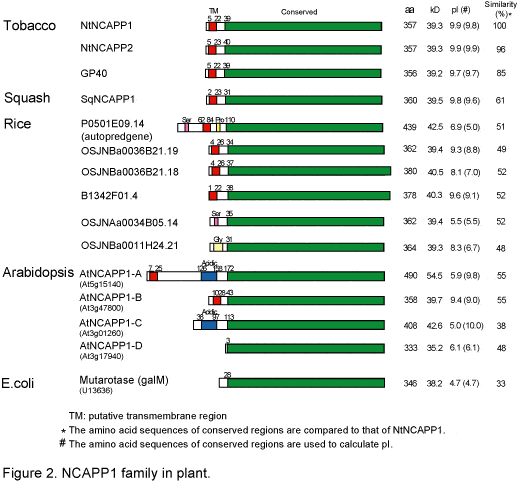
| To further investigate the molecular function of NCAPP1 in macromolecular trafficking through PD, we isolated NtNCAPP1 homologs from Arabidopsis (Figure 2). | |||
 |
|||
Four mutarotase isoforms (AtNCAPP1-A, -B, -C, and -D) were identified in the Arabidopsis genome and AtNCAPP1-A and AtNCAPP1-B contain the same putative ER-targeting trans-membrane domain identified in NtNCAPP1. Although mutarotase genes are conserved in a variety of organisms, from bacteria to mammals, our phylogenetic analysis indicated that members of the mutarotase gene family having this trans-membrane region were confined to the plant kingdom. We are currently investigating the enzymatic activity of NCAPP1 and the phenotype of T-DNA insertion lines of AtNCAPP1 genes. We will then isolate and characterize interaction partners of NCAPP1 in an effort to better define the underlying mechanism involved in NCAP function in plants. |
|||
Reference Lee, JY, Yoo, BC, Rojas, MR, Gomez-Ospina, N, Staehelin, LA and Lucas WJ (2003) Selective trafficking of non-cell-autonomous proteins mediated by NtNCAPP1. Science 299: 392-396 |
|||
